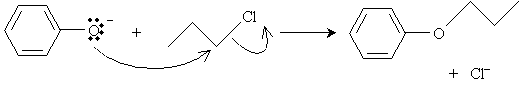Key Terms
- Aryl halide
- Phenol
- Benzyne
- Aryne
Objectives
- Study the basic properties and nomenclature of phenols and alkyl halides
- Identify reactions involving phenols and alkyl halides
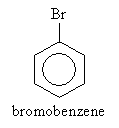
Let's look at organic compounds that are based on the benzene ring (aromatic compounds). In particular, we will look more closely at aryl halides and phenols. An aryl halide is a benzene ring in which a hydrogen atom is replaced by a halogen; we can express such molecules generally in the form ArX, where Ar is an arene and X a halogen. An example of an aryl halide (bromobenzene) is shown below.
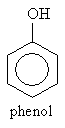
Another group of important aromatic compounds are phenols, which are defined by the presence of a benzene ring bound to a hydroxyl group. The simplest such molecule, simply called phenol, is shown below.
Nomenclature of Phenols
For most basic substituent groups (such as alkyl groups and halides), the base name for phenols is simple phenol. Thus, for example,
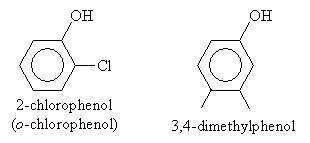
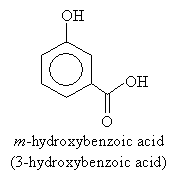
For higher-priority substituents like carboxyl groups, the molecule is named as a hydroxy-substituted benzene rather than a phenol. Thus, for instance,
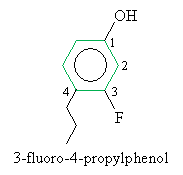
Practice Problem: Draw the molecule 3-fluoro-4-propylphenol.
Solution: The hydroxy group is located at position 1 of the benzene ring in a phenol. Thus, follow the usual procedure to place the substituent fluorine and propyl groups.
Characteristics of Aryl Halides and Phenols
Aryl halides are in many ways similar to alkyl halides. One of the major differences is the sp2 hybridization of the carbon atoms in the benzene ring, which makes the carbon atom bound to the halogen more electronegative in the case of an aryl halide versus and alkyl halide. Phenols, because of the hydroxyl group, can form hydrogen bonds. As a result, they have a higher boiling point and are more water soluble compared with similar molecules (such as toluene and fluorobenzene). In addition, phenols are relatively acidic compared with alcohols. This acidity owes to the ability of the benzene ring to stabilize a deprotonated hydroxyl group through electron delocalization (resonance forms). This phenomenon is illustrated below.
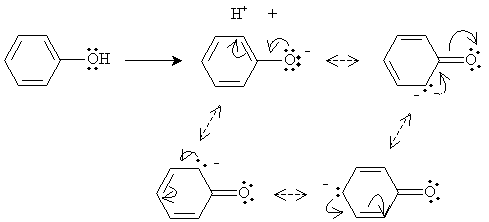
Because of the electron delocalization, phenol molecules can fairly readily exist without the proton of the hydroxyl group-in other words, they are relatively acidic.
Synthesis
Aryl halides can be synthesized by adding a halogen and an iron(III) halide to benzene. The overall reaction is shown below for the generic halogen X. The products are ArX (an aryl halide) and HX (a hydrogen halide).
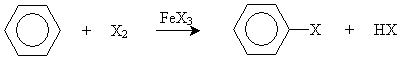
Another method of synthesizing aryl halides is by way of an aryl diazonium ion. In a reaction whose mechanism we will omit for brevity, aniline can be converted to a benzene-based diazonium ion through the following reaction, which occurs at close to 0�C.
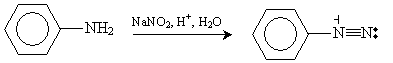
Aryl diazonium ions can then be converted into a variety of forms, including both aryl halides and phenols. For instance, adding water and heating the solution yields phenol, protons (hydronium ions), and nitrogen gas.
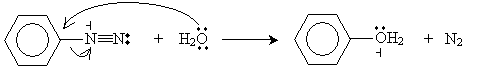
In addition, aryl halides can also be synthesized from aryl diazonium ions. Adding potassium iodide (which dissociates into potassium and iodide ions) yields iodobenzene, for instance.
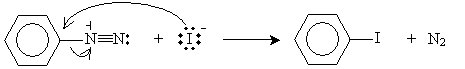
Similarly, adding copper(I) chloride or copper(I) bromide to an aryl diazonium ion can yield an aryl halide.
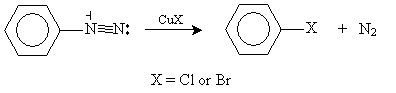
Finally, an aryl fluoride can be synthesized by adding HBF4 to an aryl diazonium ion and heating the solution, as shown below.
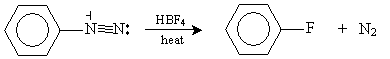
Phenol can be synthesized from, for instance, chlorobenzene. The first step of the reaction is addition of chlorobenzene to a solution of sodium hydroxide (NaOH) in water at 370�C. The sodium hydroxide dissociates, producing hydroxide ions that deprotonate the benzene ring, forming a benzyne intermediate. (As a side note, benzyne-based molecules are called arynes, just as benzene-based molecules are called arenes.)
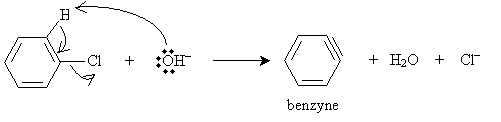
Next, a hydroxide ion attacks one of the triple-bonded carbon atoms of the benzyne molecule. (Because the benzyne molecule is symmetrical, the hydroxide ion can attack either triple-bonded carbon. The reactions yields a roughly equal distribution of the two products, which are, in this case, the same.)
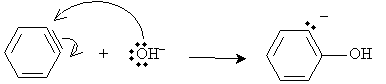
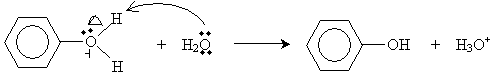
The ion intermediate in this case then reacts with water, yielding phenol and a hydroxide ion.

Practice Problem: A chemist has aniline but needs chlorobenzene. What should she do?
Solution: To synthesize chlorobenzene from aniline, the first step of one possible procedure is conversion of aniline to aryl diazonium ions, as shown below.
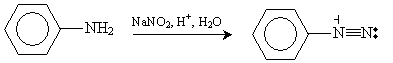
This intermediate molecule offers a number of paths to various aryl halides and phenol. In the case of our hypothetical chemist, adding copper(I) chloride (CuCl) yields the desired product.
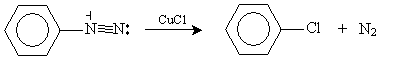
Reactions of Aryl Halides and Phenols
We saw above that an aryl halide can be converted to a phenol; here, we will consider a few representative reactions involving phenols. One such reaction is halogenation of phenol. In a nonpolar solvent such as 1,2-dichloroethane, phenol reacts with chlorine (for example) at low temperatures (0�C) to yield p-chlorophenol. The reaction mechanism, shown below, proceeds by way of electrophilic aromatic substitution.
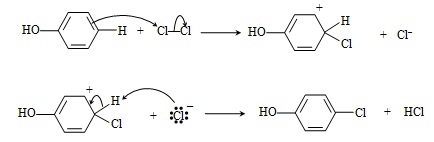
In a polar solvent (like water) at room temperature, the reaction continues by chlorinating the ortho positions of the benzene ring as well. Thus, the overall reaction in this case is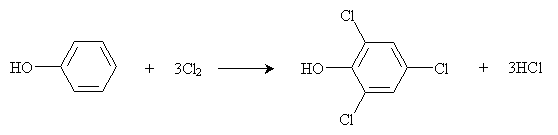
Practice Problem: Propose an explanation of why single chlorination of phenol occurs para to the hydroxyl group and why multiple chlorination occurs para and ortho to the hydroxyl group.
Solution: Generally, reactions prefer intermediate and final forms that are relatively low in energy, meaning they are more stable. In this case, chlorination of phenol yields a carbocation intermediate. Some carbocations are more stable (i.e., lower in energy) than others. One important characteristic affecting stability is electron delocalization. Consider the resonance forms for ortho, meta, and para substitution of an electrophile X.
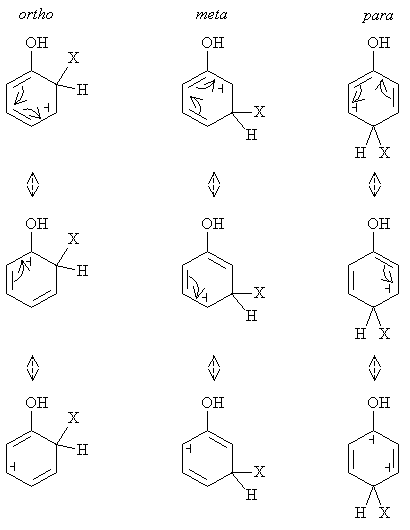
Notice that because the ortho and para substitution examples have resonance forms with the carbocation located adjacent to the hydroxyl group, they are preferred forms. This owes to the fact that the oxygen atom has two nonbonding electron pairs that can become delocalized to the carbon ring, creating more resonance forms than are possible with meta substitution.
Another example of a reaction involving phenol is synthesis of aryl ethers. In the presence of a base, such as potassium bicarbonate (K2CO3), and heat, phenol reacts with an alkyl halide to form an ether. First, the base deprotonates the hydroxyl group, creating an anion.
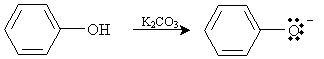
This ion can then undergo a substitution reaction with an alkyl halide as follows. In the reaction depicted below, 1-chloropropane is used as an example.