A particularly important molecule and functional group, owing to its unique stability, is benzene. Benzene is the defining component of another class of hydrocarbons called aromatic hydrocarbons (compounds) or arenes.
Key Terms
- Aromatic hydrocarbon
- Arene
- Steric strain
- Conformation
- Ortho (substitution)
- Meta (substitution)
- Para (substitution)
Objectives
- Recognize the bonding characteristics of the benzene ring that add to its stability
- Identify the names of aromatic compounds according to IUPAC rules
Aliphatic hydrocarbons include alkanes, alkenes, and alkynes (as well as their cyclic counterparts). Another class of hydrocarbons are the so-called aromatic hydrocarbons, which are also known as arenes. This class includes molecules that incorporate the six-carbon cyclic structure known as benzene:
The history of the discovery of benzene paints an interesting picture about how scientists developed theories regarding unseen objects like molecules, but we will not dwell on this history here. It is noteworthy, however, that the term aromatic or aromaticity arose from the close association of benzene-like structures to sweet-smelling plant extracts.
The Structure of Benzene
At first glance, benzene appears to be a cyclic triene (three double bonds)-one might be tempted to call it 1,3,5-cyclohexatriene. Furthermore, we can note that the structure shown above appears to have two resonance forms:
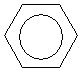
The bonds and bond lengths between the carbons do not oscillate; rather, they are halfway between the longer bond length associated with a single carbon-carbon bond of this type (146 picometers) and the shorter length of a double carbon-carbon bond of this type (134 picometers). The actual bond length between each pair of adjacent carbons in the ring is 140 picometers. Thus, each pair of adjacent carbon atoms in benzene has the character of one and a half bonds rather than one or two bonds. To help illustrate this point, benzene is often drawn as a cyclohexane ring with a circle in the middle, which helps show the delocalization of the electrons in the double bonds.
Another factor increasing the stability of benzene is the bond angles between carbons. Recall that when it forms a double bond, the carbon atom's electron orbitals hybridize to form three sp2 orbitals, leaving one unhybridized 2p orbital. The sp2 orbitals (shown in green below) form a trigonal planar arrangement, resulting in 120� angles. The 2p orbital (shown in blue) is perpendicular to the plane of the sp2 orbitals.
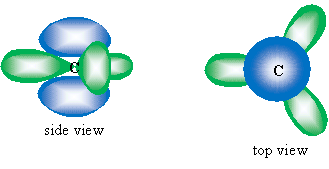
Not coincidentally, the interior angles of a hexagon are each 120�, which means that the ring structure does not create steric strain (increased energy owing to distortion of bond angles or other aspects of the atoms composing the molecule), and benzene therefore maintains a low energy, increasing its stability. The drawing below illustrates this arrangement (orbital sizes are not drawn to scale).
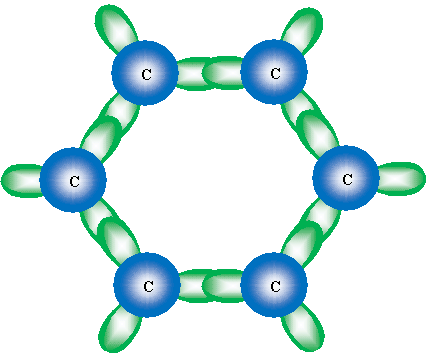
Although not an entirely accurate picture, we can view the double bonds (or, rather, the "circle" drawn in the hexagonal ring) as overlap of the unhybridized 2p orbitals. These orbitals overlap in a manner similar to the sketch shown below, which shows the overlapping 2p orbitals in the benzene structure.
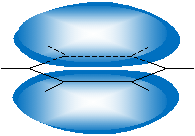
This orbital arrangement (following the VSEPR model) means that benzene is a planar molecule, unlike standard hexane. Note that, to limit steric strain and maintain the tetrahedral orientation of the sp3 hybridized orbitals, cyclohexane forms nonplanar structures like the "chair" shape (or conformation) shown below.

Another similar but less stable form (owing to greater steric hindrance) is the "boat" shape.

Characteristics of Benzene
Benzene is an important chemical with numerous uses in industry, and it was once widely used as a solvent until it was discovered to be a carcinogen. The structure and stability of benzene means that it does not undergo reactions in the way we might expect on the basis of what we have learned thus far about alkenes. For instance, although benzene can be hydrogenated like alkenes can, it is more difficult. In the presence of platinum and hydrogen gas near room temperature, benzene requires higher pressures (two or three atmospheres) to induce hydrogenation:
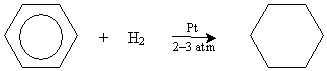
Another interesting characteristic of benzene is its interaction with electrophiles. When bromine is present with cyclohexene, electrophilic addition occurs.
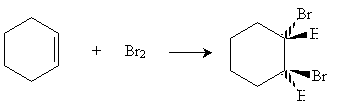
On the basis of the simple skeleton drawing of benzene as a hexatriene, one would expect a similar reaction to occur as bromine adds to benzene. This is not the case, however. By itself, bromine does not react with benzene. In the presence of an iron(III) bromide catalyst (FeBr3), however, benzene reacts with bromine, but in an unexpected manner.
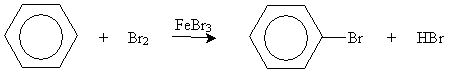
The products of this reaction are bromobenzene and hydrogen bromide; in other words, benzene undergoes substitution rather than electrophilic addition in this case. The fundamental structure of the benzene ring-six carbons with overlapping 2p orbitals-remains unchanged.
Nomenclature
As with aliphatic hydrocarbons, arenes follow IUPAC rules of nomenclature. Much of what we learned about naming hydrocarbons applies to benzene and its derivatives as well. For substituted derivatives of benzene (cases where some number of benzene's hydrogen atoms are replaced by substituent groups), we must first note that there is no preferred arrangement of the double bonds (if that representation is used). In other words, for example, the two molecules below are identical.

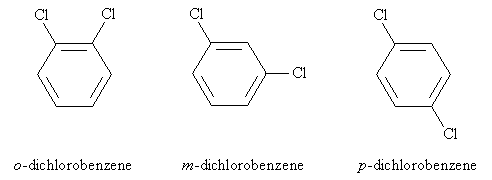
Thus, we can treat the benzene ring as a typical cyclohexane for the purposes of numbering the carbon atoms. The molecule above would then be named 1,2-dichlorobenzene. But sometimes, a slightly different approach to naming disubstituted (two substituent groups) benzene is used: 1,2-disubstitution (as in the case above) is called ortho (o) substitution, 1,3-disubstitution is called meta (m) substitution, and 1,4-disubstitution is called para (p) substitution. Following the example above, these three variations are shown below with their respective names.
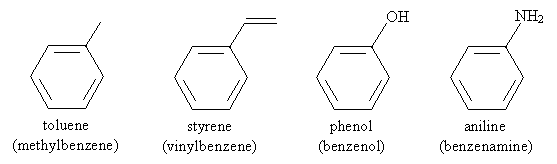
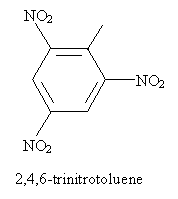
Thus, for instance, the following is an IUPAC-acceptable name for a molecule you may have some familiarity with (TNT). Note that the methyl group of toluene is assumed to be in the first position-the same applies to other base aromatic molecules (like those above) with non-systematic names.
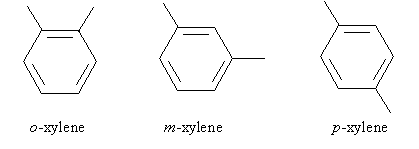
Another example is dimethylbenzene, which is commonly called xylene. But since xylene is disubstituted, more information is needed to disambiguate the molecule's form. Applying our ortho-meta-para prefixes, the following are the different types of xylene.
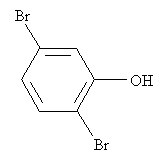
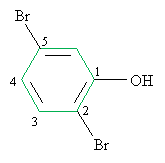
Practice Problem: Identify the correct IUPAC name for the following molecule.
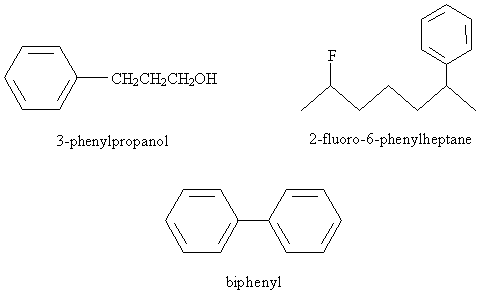
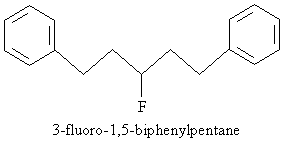
Practice Problem: Draw the molecule corresponding to 3-fluoro-1,5-biphenylpentane.
Solution: The base molecule is a pentane chain. A fluorine atom is attached at position 3, and one benzene ring is attached to the carbon at each end of the chain. The result is sketched below.