Key Terms
- Diol
- Hydroxyl group
- Thiol
- Carbonyl group
- Condensation (reaction)
- Ether
Objectives
- Learn nomenclature rules for diols
- Study reactions for synthesizing alcohols from carbonyl groups
- Review several other reactions involving alcohols
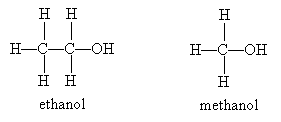
The first thing that may come to your mind when you hear the word alcohol is the intoxicating substance found in beer, wine, and liquors: ethanol. Ethanol is an alcohol that the human body can metabolize. Methanol, on the other hand, is poisonous, and ingestion can lead to blindness or death.
Ethanol is also added to most gasoline in the United States because it is relatively clean burning, although it is known to reduce gas mileage and damage engines not designed to use it.
Similar compounds that you may encounter are diols and thiols. A diol, as the name indicates, is a compound with two OH (hydroxyl) groups. A thiol is similar to an alcohol except the oxygen in the hydroxyl group is replaced by a sulfur atom. Note that sulfur sits directly below oxygen in the periodic table, meaning that it has similar properties because its valence electron shell is the same: 3s23p4 for sulfur and 2s22p4 for oxygen.
IUPAC nomenclature for diols is slightly different than that for similar compounds, but the essential principles remain. Instead of the compound ending in -ol (such as pentanol), it ends in -diol, but the preceding compound name is the full alkane name. Thus, for example, the compound below is named 2,4-pentanediol.
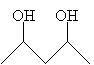
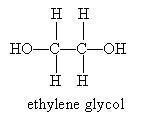
A common diol is 1,2-ethanediol, which has the more common name ethylene glycol (a component of antifreeze).
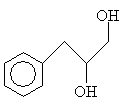
Practice Problem: Determine the IUPAC name for the following compound.
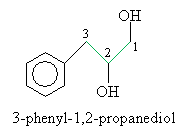
Solution: The base structure in this case is propane. The benzene ring is a phenyl group. The correct carbon numbering scheme and IUPAC name of the molecule are shown below.
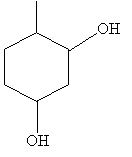
Practice Problem: Sketch the compound 6-methyl-1,3-cyclohexanediol.
Solution: The base molecule in this case is cyclohexane. Sketch the molecule, then number the carbon atoms and place the functional groups. The result is shown below.
Synthesis of Alcohols
An important method of synthesizing alcohols is reduction of a carbonyl group, which is an oxygen atom doubly bonded to a carbon atom.
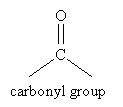
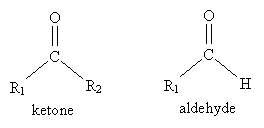
The carbonyl group is the central feature of important organic compounds like ketones, aldehydes, and esters.
Because oxygen is significantly more electronegative than carbon, however, the electrons in the double bond are not shared equally. In other words, the bond is polar: the carbon has a net positive charge (?+), and the oxygen has a net negative charge (?-).
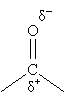
Recall that alkenes can be hydrogenated by adding hydrogen in the presence of a catalyst like platinum metal. A similar process can be used to convert a carbonyl-based molecule into an alcohol. By adding hydrogen in the presence of a metal such as platinum, palladium, nickel, or ruthenium, we can hydrogenate the carbonyl group, as shown below for the example case of formaldehyde.
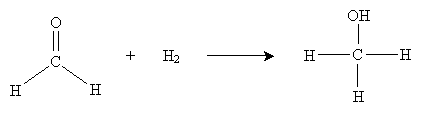
The result in this case is methanol (wood alcohol). Another approach to hydration involves metal hydrides as catalysts. The overall reaction is shown below for sodium borohydride (NaBH4) in water.
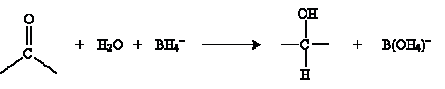
The mechanism for this reaction involves the borohydride ion interacting with the carbonyl group as follows.
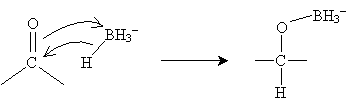
The process then repeats until the boron atom is bound to four (formerly carbonyl) oxygens. If we represent the carbonyl-based molecule as R2CO, then the result of this process is shown below.
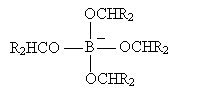
The next step involves hydrolysis as shown below.
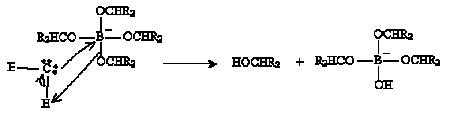
Thus, the carbonyl-based molecule is converted to an alcohol. The hydrolysis step repeats to yield B(OH)4- in addition to the alcohols.
We can also synthesize alcohols by starting with an alkene and performing a hydration reaction with water and an acid catalyst. The overall reaction in the presence of sulfuric acid is reviewed below.

Reactions of Alcohols
Alcohols can be converted to alkenes by dehydration. Alcohols can undergo a number of other reactions as well, a few of which we will survey here. A simple example is substitution to form an alkyl halide. The overall reaction for the example case of 4-hexanol and hydrogen bromide is shown below.
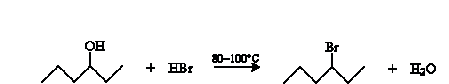
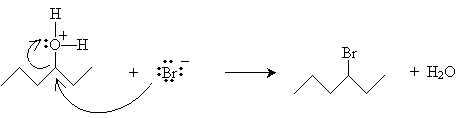
The mechanism for this reaction should be somewhat familiar. It begins with hydrogen bromide acting as a Br nsted-Lowry acid.

Note that if the hexane chain were to dissociate a water molecule at this point, it would form a carbocation at a carbon atom that has only two substituent alkyl groups, meaning this carbocation would be extremely high in energy. Thus, instead of undergoing an SN1-type (single-molecule) reaction, it undergoes an SN2-type (two-molecule) reaction.
Another type of reaction involves condensation of an alcohol into an ether. An ether is an oxygen atom with two substituent alkyl groups. (We will discuss ethers in more detail in the next chapter.) The condensation reaction is conducted in the presence of acid and heat. For instance, consider the case of ethanol in the presence of sulfuric acid. Ethanol acts as a Br nsted-Lowry base, accepting a proton from sulfuric acid.
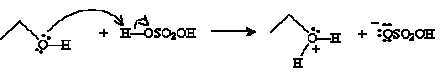
The next step involves another ethanol molecule.
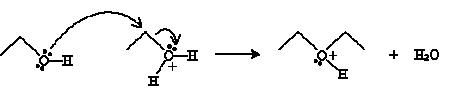
And finally, another ethanol molecule undergoes an acid-base reaction with the cation product above.
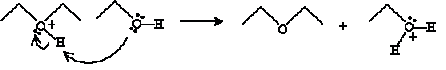
The products of this reaction are diethyl ether and water.
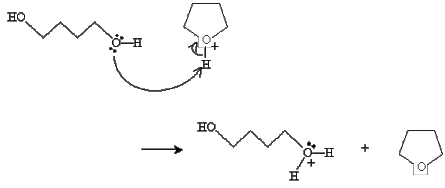
Practice Problem: A diol can undergo a reaction similar to the condensation reaction discussed above to form a cyclic ether. Propose a mechanism for the formation of oxolane (or tetrahydrofuran), shown below, from 1,4-butanediol in the presence of sulfuric acid and heat.

Solution: The reaction mechanism in this case is almost identical to that of the discussion above, but it involves a single alcohol molecule instead of two. To start, one of the hydroxide groups reacts with sulfuric acid.
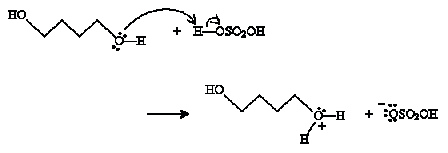
Next, the hydroxide group at the other end of the chain closes the ring and a water molecule is dissociated.
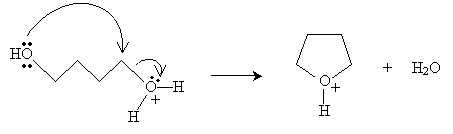
Finally, a diol molecule undergoes an acid-base reaction with the cyclic structure to form the final product.