This article reviews reactions that involve replacement of one functional group by another. These substitution reactions are instigated by nucleophiles-"nucleus-seeking" compounds.
Key Terms
- Nucleophile
- Nucleophilic substitution
- Leaving group
- SN2 mechanism
- SN1 mechanism
- Steric hindrance
- Nucleophilicity
Objectives
- Recognize the relationship between nucleophiles and Lewis bases
- Identify mechanisms for nucleophilic substitution reactions
- Rank chemicals on the basis of their nucleophilicity
The reactions of electrophilic addition involve a Lewis acid (an electron-seeking species) interacting with the double bond of an alkene (or the triple bond of an alkyne) to add substituent groups to the molecule. The opposite (in some sense) of an electrophile is a nucleophile: a "nucleus-seeking" species. Nucleophiles can also be viewed as electron pair donors, or Lewis bases. These species can react with alkyl halides (hydrocarbons with halide groups) to undergo a nucleophilic substitution reaction. We will consider the mechanisms and some of the factors involved in these reactions.
Recall that halogens are highly electronegative (but decreasingly so with increasing atomic number): for instance, fluorine is the most electronegative element. Thus, for instance, electrons in a hydrogen halide molecule tend to be attracted more to the halogen than to the hydrogen, resulting in a dipolar molecule (that is, a molecule with a dipole moment). In solution, hydrogen halides readily dissociate the hydrogen nucleus (proton), forming halide ions. In other words, hydrogen halides are strong acids, and the conjugate base, the halide ion, is very weak. Thus, when a Lewis base (an electron donor) interacts with an alkyl halide, the following substitution can take place, where R represents the alkyl group, X the halogen, and B the Lewis base (an ion, in this case).

The nucleophile (or Lewis base) B donates an electron pair to the alkyl group, and the halide group takes the electrons that bond it with the alkyl group when it leaves (the halogen in this case is called a leaving group). Although not all nucleophiles are anions, many are, including the following examples:
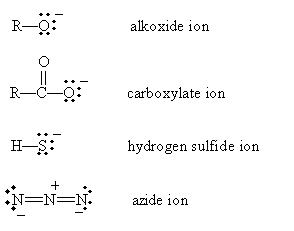
The halogens with higher atomic numbers are better leaving groups because of their weaker bonds with alkyl groups. Thus, for instance, iodine leaves an alkyl group more readily than does fluorine.
SN1 and SN2 Mechanisms
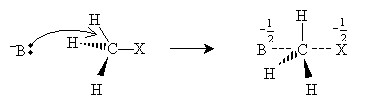
The halide ion then dissociates from the transition molecule, completing the substitution reaction.
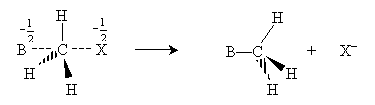
Note that the way in which the nucleophile attacks causes the hydrogen atoms bonded to the carbon to invert their positions like a broken umbrella. Although the symmetry of this example means that this change has no effect on the structure of the molecule, it can have an effect in some cases. Also note that hydrogen atoms are fairly small, leaving plenty of room for the nucleophile to approach the carbon atom bonded to the halogen. As this carbon atom becomes more highly substituted, however, the nucleophile will have an increasingly difficult time approaching; thus, more highly substituted carbon atoms are less reactive. For instance, methyl bromide is more reactive than ethyl bromide, which is more reactive than 2-methyl-2-bromopropane. The "crowding" that limits reactivity because of increasing substitution is called steric hindrance.
The SN1 mechanism involves a carbocation stage that readily reacts with a nucleophile. The alkyl halide is shown with three substituent groups (R) for the sake of example, but the carbon atom may (theoretically) have any amount of substitution.
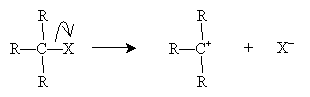
The nucleophile B- then attacks the carbocation intermediate.
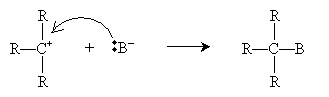
When the carbon atom bound to the halogen is more highly substituted (as in the example above), the SN1 mechanism tends to dominate, whereas the SN2 mechanism tends to dominate when that carbon atom is less highly substituted. Thus, for instance, methyl halides undergo SN2 substitution almost exclusively, whereas "tertiary" (R3CX) alkyl halides undergo SN1 substitution almost exclusively.
Practice Problem: By what mechanism will a nucleophile replace the halogen in 2-methyl-2-chloropentane?
Solution: First, draw the molecule 2-methyl-2-chloropentane.
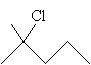
For a nucleophile to replace the chlorine atom via the SN2 mechanism, it must attack the carbon in position 2 from the direction opposite the chlorine. Because this carbon has three substituent alkyl groups, steric hindrance makes this mechanism virtually impossible. When 2-methyl-2-chloropentane forms a carbocation intermediate, however, the substitution is more likely. This corresponds to the SN1 mechanism.
Practice Problem: Cyclohexyl bromide is present with hydrogen sulfide ions. What are the products of the ensuing reaction?
Solution: A nucleophilic replacement reaction occurs, with the results shown below.
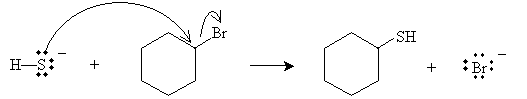
Nucleophilicity
Nucleophiles and Lewis bases are similar (and the same in many cases), and since we are able to rank bases according to their relative strengths, why not rank nucleophiles according to how nucleophilic they are? The "strength" of a nucleophile is called its nucleophilicity, and we can identify several characteristics of nucleophiles that help us determine relative nucleophilicity.
Since nucleophiles overlap with Lewis bases so closely, the temptation is to rank nucleophilicity according to basicity. To some extent, this is a valid approach: as long as the nucleophilic atom is the same for two compounds (for instance, oxygen)-or, more broadly, as long as the nucleophilic atoms are in the same row of the periodic table-then the more basic compound is also the more nucleophilic compound. For instance, the ion formed when methanol dissociates a proton is more basic than water, and therefore, it is more nucleophilic.

For nucleophilic atoms in different rows of the periodic table, however, this approach does not work in solutions. Recall that crowded molecules can hinder attack by nucleophiles-similarly, ionic nucleophiles (such as halide ions) can be hindered by ion-dipole attractions. For instance, a fluoride ion, which is negatively charged, will attract water molecules, which are dipolar. This attraction, and therefore "crowding" by water, can hinder fluoride from attacking an alkyl halide. This ion-dipole attraction lessens for larger atoms (for example, iodine), meaning that a higher atomic number indicates greater nucleophilicity (within a given column of the periodic table).
Practice Problem: Determine which of the following is the most nucleophilic.

Solution: The nucleophilic atoms are sulfur (S) and oxygen (O), which are in the same column of the periodic table. Since sulfur is the larger atom (and thus less susceptible to crowding because of ion-dipole forces), and therefore it is more nucleophilic. Thus, hydrogen sulfide is the more nucleophilic species.