Key Terms
- Carbohydrate
- Ketose
- Aldose
- Monosaccharide
- Disaccharide
- Oligosaccharide
- Stereoisomer
- Cyclic hemiacetal
Objectives
- Identify basic ketoses and aldoses (carbohydrates)
- Draw Fischer projections for carbohydrates
- Understand how carbohydrates interconvert between linear and cyclic forms
Many organic molecules (particularly those involved in biology) are much more complicated than just a five- or six-carbon chain with one or two functional groups. Many organic molecules are highly complex polycyclic structures that can even be difficult to draw clearly. Think, for example, of DNA-this is nothing more ("nothing more"!) than a very complex organic molecule, but drawing it in the same manner would be extremely difficult.
Despite the complexity of larger organic molecules-particularly those involved in biological processes-a study of organic chemistry would not be complete without at least a cursory introduction to some of these structures. We will very lightly consider carbohydrates, which include what are commonly called sugars. Carbohydrates are critical energy sources for living organisms.
Introduction to Carbohydrates
Fundamentally, a carbohydrate is defined as an aldehyde or ketone that has multiple hydroxy1 groups. A point worth noting from the start of our discussion is that carbohydrates are chiral molecules. A molecule is chiral if it has more than one configuration for which the chemical formula and structure are the same, but the arrangement in three-dimensional space differs. For example, the two molecules below are identical except for the arrangement of the atoms around the chirality center.

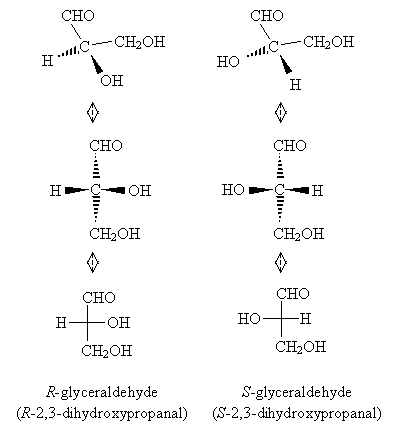
In the same way, carbohydrates are chiral molecules. Consider the simple carbohydrate 2,3-dihydroxypropanal; this molecule has a common name of glyceraldehyde.
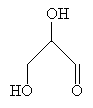
The chirality center of this molecule is the middle carbon, and the molecule has two enantiomers. We can draw a representation of the three-dimensional structure of the enantiomers as follows. (Note that we cannot superimpose one enantiomer precisely on the other, no matter how we turn it in three-dimensional space.)
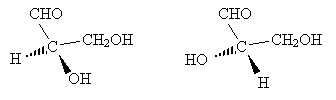
We can also draw the corresponding Fischer projections.
As mentioned above, carbohydrates can be either ketones or aldehydes; in the former case, they are called ketoses, and in the latter case, they are called aldoses. Carbohydrates are further divided into the classes of monosaccharides, disaccharides and oligosaccharides. A monosaccharide is a carbohydrate that cannot undergo hydrolysis to form smaller carbohydrates. Following this logic, a disaccharide yields two monosaccharides on hydrolysis, and an oligosaccharide yields two or more monosaccharides on hydrolysis.
Focusing on aldoses in particular, these molecules are further named according to the number of carbon atoms in the chain. For instance, glyceraldehyde is an aldotriose. (Note that the name is aldo- + (number prefix) + -ose.) Thus, aldotetroses, aldopentoses, and aldohexoses are aldoses with four, five, and six carbons, respectively.
Part of the common system for naming aldoses involves D-L notation. The 'D' designation indicates similarity to R-glyceraldehyde, and the 'L' designation indicates similarity to S-glyceraldehyde. In particular, the similarity relates to the highest-numbered carbon chirality center in the aldose, where the numbering scheme is the same used in IUPAC nomenclature. Consider, for example, the aldotetrose shown below, where the carbon chain is numbered in accordance with IUPAC rules (i.e., in this case beginning with the highest-priority functional group-the carbonyl group).
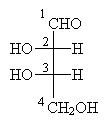
The highest-numbered chirality center is carbon 3. Note that carbon 4 is not a chirality center: if we treat everything above carbon 4 in the diagram as a single functional group R, then the molecule looks like the following.
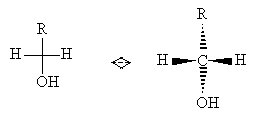
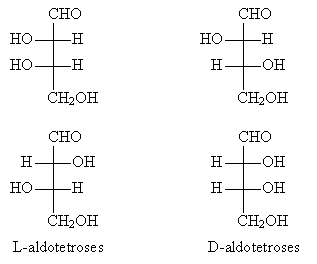
But the mirror image of this molecule has the same configuration as the original, so the carbon atom shown explicitly above is not a chirality center. Back to our example aldotetrose molecule, we see that the arrangement of groups at the highest-numbered chirality center is the same as that for S-glyceraldehyde; thus, it is an L-aldotetrose. The diagram below compares the two variations.
To further differentiate aldotroses, each has a nonsystematic name. We will not spend much time learning these names (since this would just be an exercise of memorization), but it is worthwhile to note that this is the naming structure for carbohydrates.
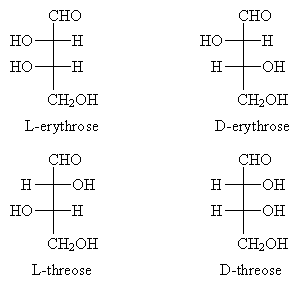
A carbohydrate whose name you may recognize is shown below in its D and L forms. Note that these molecules are aldohexoses.
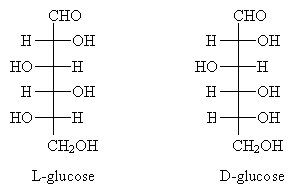
Note that glucose is more formally called 2,3,4,5,6-pentahydroxyhexanal. (Technically, it is one possible stereoisomer of this molecule. Stereoisomers are molecules that have the same composition but differ in the spatial arrangement of their constituent atoms. )
Practice Problem: Draw the Fischer projection for the carbohydrate below.
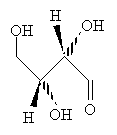
Solution: First, we must arrange the molecule so that we can easily convert it to a Fischer projection. Notice that once we rotate one of the central carbon atoms as shown below, we can draw the molecule in a form that allows easy conversion to a Fischer projection.
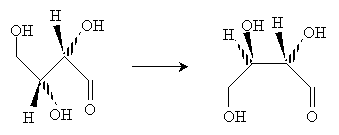
Reorient the molecule once more to the form below.
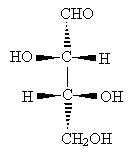
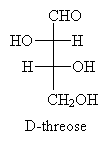
Now, draw the Fischer projection. This molecule is D-threose.
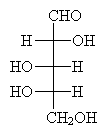
Practice Problem: Determine if the molecule below is an L or D aldopentose.
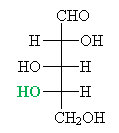
Solution: Look at the highest-numbered chirality center-in this case, the fourth carbon down the chain-and compare it to glyceraldehyde. Because the hydroxyl group in on the left side of the Fischer projection, this molecule is an L-aldopentose.
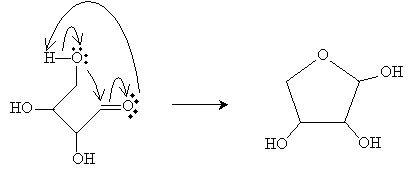
Cyclic Carbohydrates
The carbonyl group and terminal (end of the carbon chain) hydroxyl group can react to convert a linear carbohydrate (such as the aldoses above) into a cyclic carbohydrate. The resulting ring is called a cyclic hemiacetal. Cyclic forms are most common with four- and five-carbon chains. Consider the case of threose (2,3,4-trihydroxybutanal). The rearrangement of electrons of the linear molecule yields an ether-like ring structure.